R&D
Science
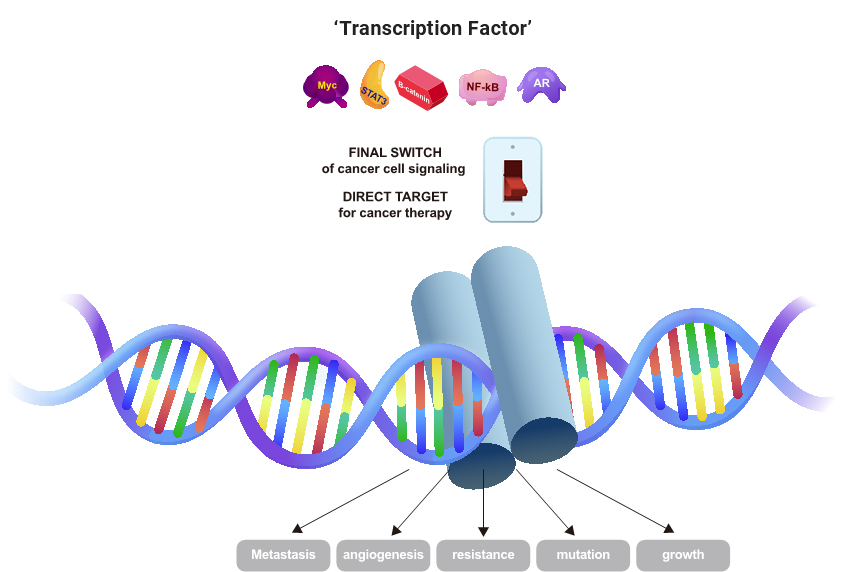
Why develop Transcription Factor inhibitors?
Transcription Factors (TFs)' are commonly deregulated in almost all human cancers. The main causes of the altered expression of TFs are chromosomal translocations and TF encoding genes that suffer from amplification or deletion, point mutations, etc. These mechanisms directly alter the TF's status to gain or loss-of-function. Other main driving mechanisms that affect the TF regulation in cancer include mutation of TF cofactors and numerous oncogenic signal transduction cascades, leading the gene expression status to favor the cell transformation. Countless pathways and mechanisms of TF deregulation in cancer show that TFs play a central role in aberrant gene expression in cellular transformation and are a major class of cancer cell dependencies. As we already learned from successful cases of drugs that target nuclear hormone receptors, the targeting of TFs can be highly effective in treating certain cancers.
Why Interrupt Protein-DNA Interaction?
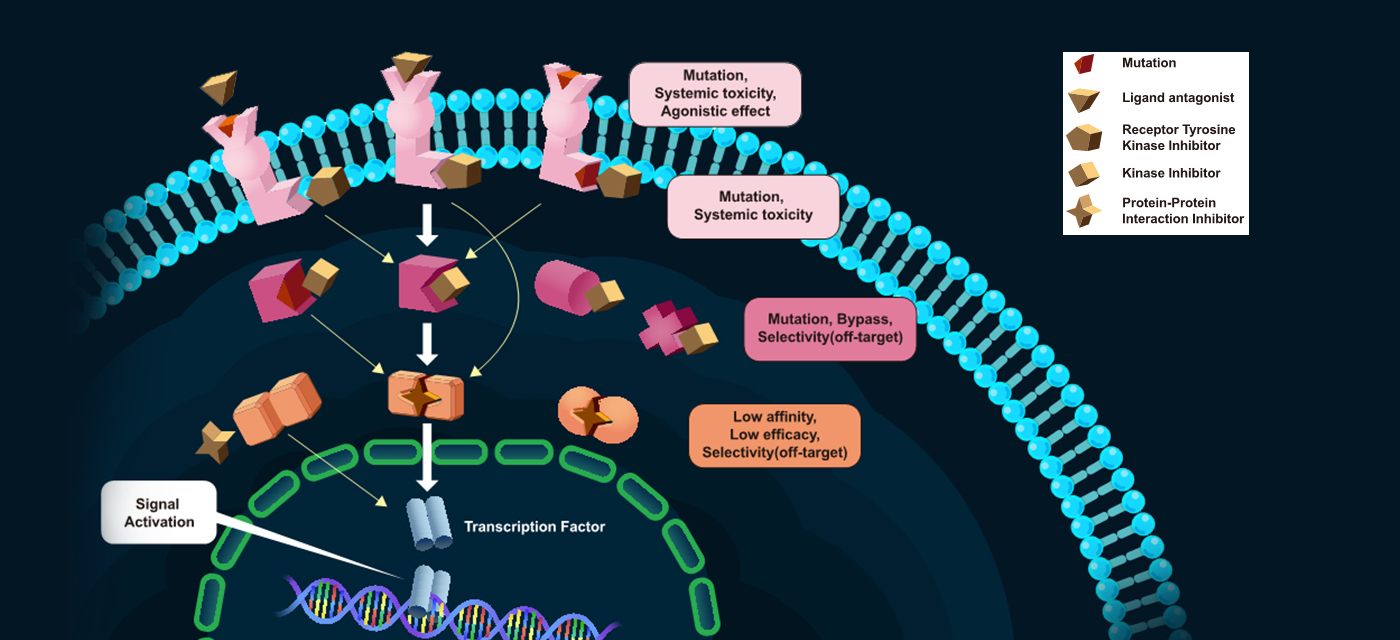
Traditionally, researchers have been trying to target the upstream pathway to ultimately regulate TF activity or TF expression level.
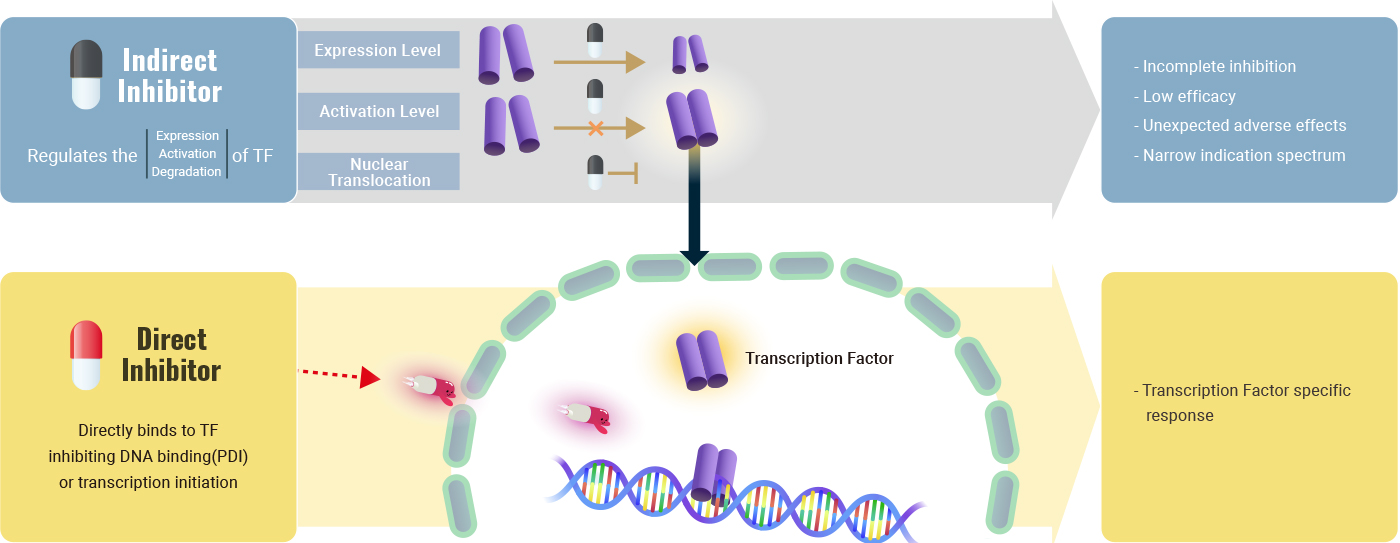
But these days, we are employing multiple approaches to directly target transcription factor activity, mostly in the preclinical stage. Their MOA includes (1) inhibition of protein-protein interaction (PPI) like TF–cofactor or TF dimerization, (2) inhibition of Transcription Factor protein–DNA binding interaction (PDI), (3) decreasing the level of TF protein by promoting proteasomal degradation via direct ubiquitylation or, currently, via PROTAC technology, etc.
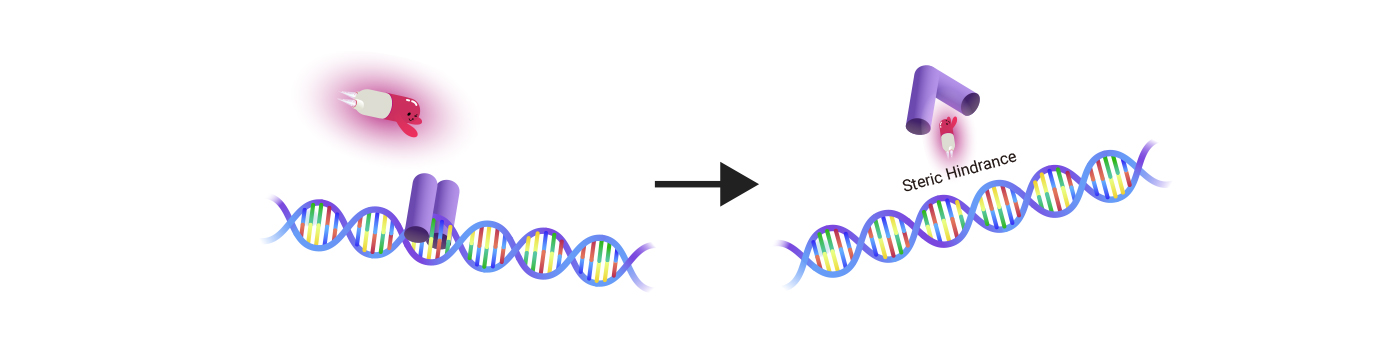
Incurix is adopting the PDI over the other available strategies of direct TF inhibition. Even though Transcription Factor proteins are notorious for being an undruggable target, TFs have strong points where we can find impactful benefits once we find success:
- Sequence-specific DNA binding site for each TF doesn't undergo key sequence changes (mutation). Even if this happens, then the cancer will cure itself since the Transcription Factors with mutations at the DNA binding sites will not bind to the consensus DNA sequence. This also applies in reverse cases. -> Incurix's optimized PDI strategy is without possible drug resistance due to the DNA mutation which is common to most small-molecule drugs.
- Incurix's PDI drugs don't destroy the TF proteins. It only inhibits and prevents TFs from binding to DNA strands. Hence, there is no need for concern about unnecessary TF expression surges or feedback loops, provoked by the sudden decrease of TF proteins due to protein degrading or expression-regulating drugs.
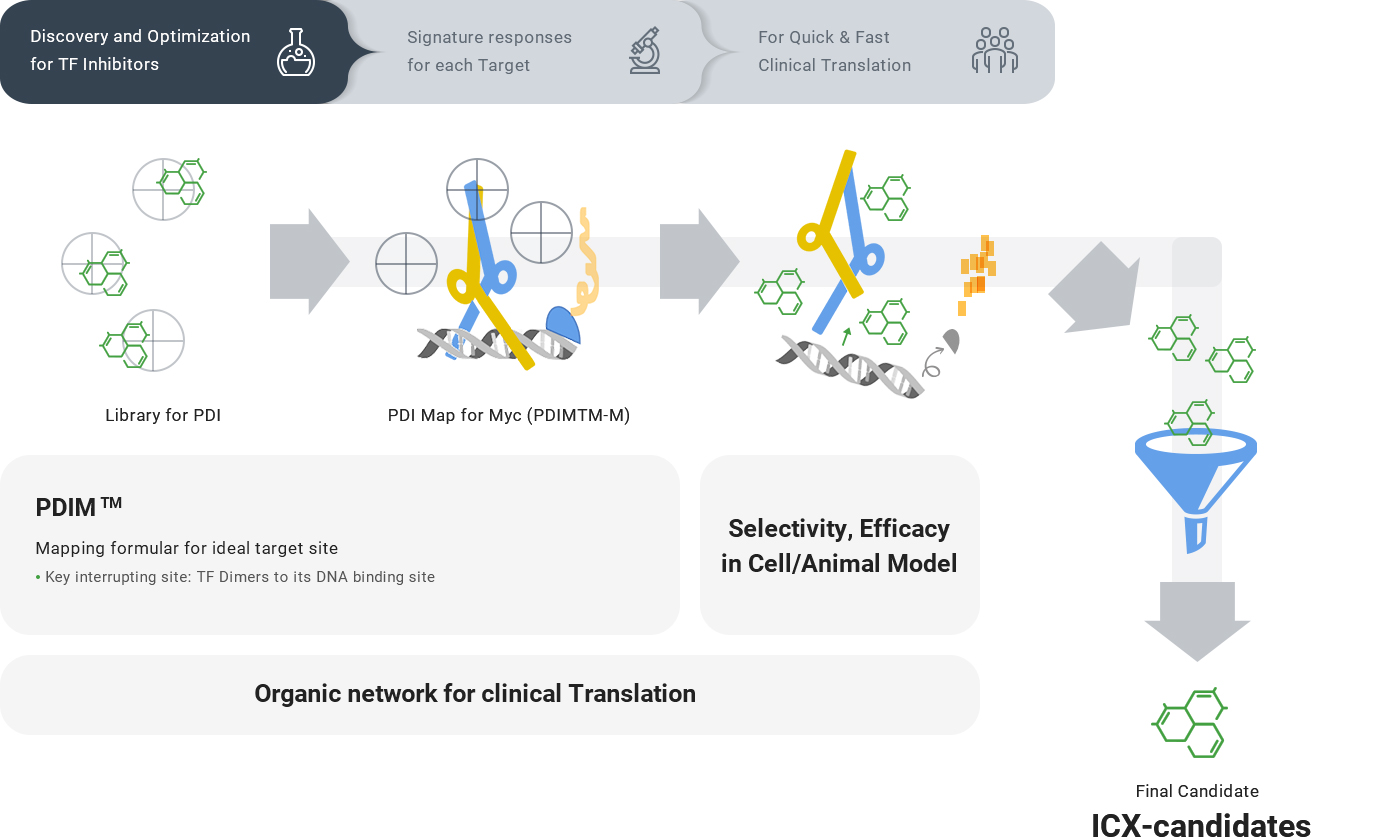
Platform Technology
We start with the knowledge that a target Transcription Factor, with its unique fluctuating and unpredictable structure, becomes rigid upon binding to its consensus DNA sequence. We map the points of each TF protein when and where it becomes druggable. To identify these substructures and target the TF target regions, Incurix has developed a comprehensive drug validation toolkit for two areas:
- Where possible, we adopt the conventional tools of small-molecule drug discovery to address Transcription Factor targets.
- Where needed, we use newly invented, proprietary tools to establish a broad structure-based drug design platform for RNA.
The result is an end-to-end TF-targeted drug discovery platform used to identify small molecules that bind to and inhibit the TF to predictably impact its transcriptome profiles.
The goal of our R&D strategy is to find the ideal TF inhibitor beyond the standardized active evaluation criteria through the core technologies. We set the criteria according to the target biology of each Transcription Factor in the cancer cell environment.
- Key interrupting site: TF Dimers to its consensus DNA sequence
- Optimal Drug status for given TF behavior in cancer: binding affinity, optimal half-life
- Specific candidate selection which generates each given TF's unique transcriptome responses: Validation system that differentiates and selects the right candidates for the given TF's
- Translational research: Cancer cell line panel and NCC's cancer patient-derived tumor cells